
envafolimab(kn035)* is an investigational pd-l1 antibody currently being evaluated in broad clinical studies in the us, china, and japan. it is created by a fusion of the of pd-l1 domain antibody with the fc domain of a regular antibody. compared with conventional pd-(l)1 antibodies currently approved and under development, kn035’s unique design makes a differentiated and competitive profile: better tumor tissue penetration in animal model studies, subcutaneous injectable, high affinity and stability, low immunogenicity, mutated fc domain to eliminate adcc/cdc activity, and comparable in vivo half-life.

kn035 : crystal structure
current pd-(l)1 therapies in the market require frequent intravenous administration, a major challenge to patient compliance and convenience. furthermore, there is a growing population of cancer patients who need more user-friendly pd-(l)1 therapies:
kn035 is the first approved subcutaneous pd-l1 antibody therapeutics, on the market. it can be injected easily without the need for intravenous (iv) injection, therefore significantly shortens the administration time. kn035 has the potential for home-based use, which will be more convenient use for tumor patients and improve the quality of life for patients. kn035 has obtained the u.s. fda's orphan drug designation for advanced biliary tract cancer.


asco 2020 poster:envafolimab (kn035) in advanced tumors with mismatch-repair deficiency
asco 2019 poster:phase i study of kn035, the first subcutaneously administered, novel fusion anti-pd-l1 antibody in patients with advanced solid tumors in china
esmo 2018 poster:phase i study of kn035, a novel fusion anti-pd-l1 antibody administered subcutaneously in patients with advanced solid tumors in the usa
kn046 is the world's first recombinant humanized pd-l1/ctla-4 bispecific antibody independently developed by jiangsu alphamab. its innovative designs include: a proprietary ctla-4 domain antibody with a significantly improved safety profile; a bispecific antibody fused with pd-l1 antibody; engineered to target the tumor microenvironment with high pd-l1 expression, and treg clearing function.

kn046 : crystal structure
there are about 20 clinical trials of kn046 in different stages covering more than 10 types of tumors including nsclc, pancreatic cancer, thymic cancer, hcc, escc and tnbc in australia, the us and china. the results of these clinical trials have shown an advantage in survival for patients. alphamab oncology has received fda clearance to enter phase ii trial of kn046 based on the clinical results in china and australia. moreover, kn046 has obtained the u.s. fda's orphan drug designation for thymic epithelial tumor in september 2020. four pivotal clinical trials are currently being conducted, among which the interim analysis of the phase iii clinical study of kn046 combined with chemotherapy as the first-line treatment of nsclc successfully met the prespecified pfs endpoint.



wclc 2020 mini oral report:preliminary safety, efficacy results of kn046 (bispecific anti-pd-l1/ctla4) in subjects with rare thoracic tumors
wclc 2020 poster:a phase ii study of kn046 (a bispecific anti-pdl1/ctla-4) in patients (pts) with metastatic nonsmall cell lung cancer (nsclc)
asco-gi 2020 poster:the preliminary efficacy and safety of kn046 plus concurrent chemoradiation therapy in recurrent and metastatic esophageal squamous cell carcinoma
asco 2020 poster:the preliminary efficacy and safety data of kn046 (bispecific anti-pd-l1/ctla4) in patients failed on prior immune checkpoint inhibitors therapy
kn026* is an anti-her2 bispecific antibody invented by alphamab oncology using the proprietary fc-based heterodimer bispecific platform technology called crib (charge repulsion induced bispecific). kn026 can bind two non-overlapping epitopes of her2 simultaneously, leading to a dual her2 signal blockade. kn026 has demonstrated superior efficacy compared with trastuzumab and pertuzumab in combination, such as increased binding affinity, as well as better tumor inhibition in her2-positive tumor cell lines. additionally, kn026 has also shown inhibitory effect on tumor cells with medium or low her2 expression or trastuzumab-resistant cell lines.

kn026 : crystal structure
kn026 received ind approval from the national medical products administration (nmpa) of china and u.s. food and drug administration (fda) in 2018. currently, a number of clinical trials are underway in china and the united states, including breast cancer, gastric cancer/gastroesophageal junction cancer, etc. two registration clinical studies of kn026 combined with chemotherapy and kn026 combined with kn046 without chemotherapy in the treatment of gastric cancer are in progress.

the results of prior clinical studies showed that kn026 has good efficacy and safety profiles, even in heavily pretreated patients with her2-positive breast cancer..


asco 2022 poster:a phase ii study evaluating kn026 monotherapy in patients with previously treated, advanced her2-expressing gastric or gastroesophageal junction cancers
asco 2021 abstract:the preliminary efficacy of kn026 (anti-her2 bsab) in advanced gastric and gastroesophageal junction cancer patients with her2 expression
the therapeutic effect of her2 targeted therapy depends on the body's adaptive immune response. therefore, the combination of immune checkpoint inhibitors such as pd-1/pd-l1 and ctla-4 can synergistically enhance the anti-tumor effect of her2 therapies. the combination of her2 targeted drugs and pd-1 antibody has shown great efficacy in her2-positive breast cancer and gastric cancer, including a phase 2 trial of triple combination regimen (pembrolizumab,trastuzumab,and chemotherapy) as first-line therapy for her2-positive metastatic gastric cancer showing 91% orr,13.0 months median pfs and 27.3 months median overall survival. the phase ⅲ trial of this triple combination regimen (keynote-811 study) is ongoing.

kn026 and kn046 have shown good safety, tolerability and anti-tumor efficacy in mono-drug clinical trials. the combination of kn026 plus kn046 demonstrated preliminary good efficacy, safety, and tolerability in an open label, multi-center clinical study in patients with advanced her2-expressing solid tumors and have failed the standard of care treatment. the u.s. food and drug administration (fda) has granted orphan drug designation (odd) to kn026 in combination with kn046 for the treatment of her2-positive or low expressing gastric or gastroesophageal junction cancer. currently, alphamab oncology is conducting a phase iii pivotal trial of kn026 plus kn046 in the first-line treatment of her2-positive locally progressive unresectable or metastatic gastric and gastroesophageal junction cancer without chemotherapy.


sitc 2020 poster:
kn019 is a biosimilar of belatacept (nulojix®), an immunosuppressive agent approved for prophylaxis of organ rejection in adult patients receiving a kidney transplant. as a fusion protein composed of the fc-fragment of a human igg1 immunoglobulin linked to the extracellular domain of ctla-4, belatacept acts as a selective t cell co-stimulation blocker. belatacept’s superior efficacy profile has been demonstrated in long-term outcome studies*.
 kn019 : crystal structure
kn019 : crystal structure
belatacept is an improved version of abatacept (orencia®) with higher potency. orencia is approved for rheumatoid arthritis, idiopathic arthritis, and psoriatic arthritis, and it achieved global sales of about us$2.7 billion in 2017.
kn019 has started phase ii trial for rheumatoid arthritis in august 2019 and will expand to oncology-related indications in the future.

due to its structure of a fusion protein with complex glycosylation, belatacept is very difficult to manufacture as the biosimilar product. so far, few companies are able to develop a competitive biosimilar of belatacept. in contrast, kn019 has demonstrated robust cmc and superior quality in multiple batches of large-scale production.


kn052 is a pd-l1/ox40 bispecific antibody developed by alphamab oncology using the company's bispecific antibody platform. it can simultaneously bind pd-l1 and ox40, effectively reversing tumor induced immune inhibition by blocking the pd-l1/pd-1 pathway and promoting the immune response by agonizing ox40. kn052 prevents the immune escape of tumor cell, on the other hand, it activates ctl t cells and attenuates treg-mediated immunosuppression. through synergistic mechanisms, kn052 is expected to exert strong antitumor efficacy.

kn052:product structure
pd-l1 antagonist and ox40 agonist activity in one molecule;tandem structure for antigen binding domain arrangement to attenuate anti-ox40 toxicity;wildtype igg1 fc with full fc function to enable depletion of tregs overexpression ox40. ox40 is a member of key class of t cell costimulatory molecules. ox40, through interaction with ox40l, plays key role in survival and amplification of effector t cells and memory t cells and induce cytokine secretion. it can be used as an adjuvant in combination with tumor vaccines and cell therapy.

in preclinical studies, kn052 showed significantly stronger activity than single antibody or in combination. in february 2022, the ind for kn052 was approved by the national medical products administration (nmpa) to initiate phase i clinical trials in china. the first patient was dosed in the kn052-chn-001 phaseⅰtrial of kn052 in june 2022.


jskn003 is an anti-her2 bispecific antibody-drug conjugate (adc), which is developed inhouse with proprietary glycan-specific conjugation platform. through enzyme catalysis and click chemistry, the payloads are site-specifically linked to glycan on fc. the glycan structure is precisely controlled via process engineering which renders consistent dar with more favorable stability. upon binding with her2, jskn003 induces clustering of the receptor and triggers extensive internalization the jskn003-her2 complex. the internalization leads to release of payload that kills tumor directly or via and by stander effect.
comparing with other adc drugs, jskn003 induces faster and more intense endocytosis , which leads to stronger bystander effect in her2-expressing tumors. meanwhile the better serum stability ensures much wider therapeutic window. for example, jskn003 shows better safety profile in preclinical study and similar tumor killing activity with enhertu (ds-8201) in both high and low her2 expression model (cdx pdx).
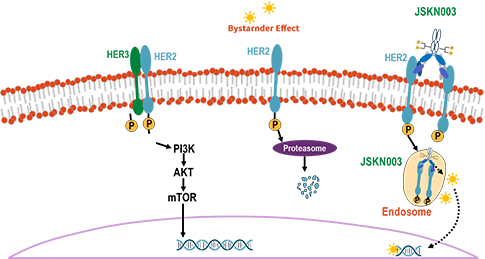
an open-label, dose-escalation, first-in-human phase i trial in australia is ongoing to assess safety and tolerability in patients with advanced or metastatic solid malignancies and to determine the maximum tolerated dose /rp2d. the ind of jskn003 in china has also been approved by nmpa.

